Energy Diagram Covalent Bond
Covalent bond Fourth grade gc: august 2013 Covalent bonding
89. CHEMICAL BONDING (36)- Covalent Bonding(35) – Molecular Orbital
8.9: covalent bond properties: order, length, and energy Covalent bonding – gcse chemistry (combined science) aqa revision Covalent bonding chemistry ch4 gcse methane water combined science aqa h2o
Energy potential bond diagram covalent formation waals der van bonds diagrams graph binding physics
Covalent bond for example water molecule h2o vector imageAtoms covalent energy bonding chem two bond graph potential distance internuclear bonds vs hydrogen chemistry line single first polar labeled Covalent bonds bond chemical types ionic four atoms between molecule two hydrogen online fsCovalent bonding.
Polar covalent bonds nonpolar kovalen ikatan molecule hydrogen atoms molecules h2o atom chemical electronsCovalent bond- definition, properties, types, examples Alcohol chemical covalent ethyl energy bonding models molecular formula bonds physiology ethanol stock powers structural molecules leonid andronov shutterstock waysChemical atoms molecule bonds formed.

Bond covalent energy potential bonding atoms theory two lewis diagram between formation adichemistry difference model when general
Energy and covalent bond formationCovalent types properties bonding electrons atoms stable Chemical bonds · anatomy and physiologyCovalent bond.
Chapter 5.6: properties of polar covalent bondsIon-ion interactions – the credible hulk 10 notable differences between ionic and covalent bonds : currentPolar bond.

How does potential energy change when a chemical bond is formed?
Covalent energy bond h2 virial 2008 analysis interaction formation graph csbsju december eduWater bonds molecule hydrogen structure covalent polar chemical chemistry anatomy oxygen atoms polarity properties physiology figure biology bond atom electrons The covalent bondEnergy ion ionic bonding covalent chemical versus chemistry interactions lattice bond distance break when system potential interaction diagram internuclear between.
Bonding chemical ionic covalent molecular crystals orbitals types crystal atoms molecules interactions forces science compounds ions atkins he2 these89. chemical bonding (36)- covalent bonding(35) – molecular orbital Covalent bondingOrbital molecular bonding nitrogen molecule theory covalent chemical.

Potential energy two bond atoms covalent hydrogen bonding chemistry diagram between ionic chemical versus lewis structures distance represent internuclear interaction
Inorganic chemistryPotential energy diagrams for formation of bonds Covalent bonds bond formed atoms electrons between two which valence chemical nonmetals ppt pair powerpoint presentation alwaysBond covalent h2o water molecule example vector.
Lewis structure diagram molecule electron bond covalent oxygen bonds molecular double orbital dots ck atoms two electrons atom align sharedCovalent bonds atoms when energy hydrogen bonding losing amount combine they gif Covalent bondingHow can i represent enthalpy in a potential energy diagram?.

Bond energy length potential covalent energies atoms lengths two order molecule breaking when why distance diagram bonds curve formation between
Covalent bondsCovalent bonds ionic polarity bonding covalente atoms electron electronegativity nonpolar polaire libretexts liaison structures molecules molecular chapter electrostatic electrons purely Chemical energy in physiologyCovalent bonding.
Chemical bondingEnergy potential bond atoms covalent formation bonds two hydrogen distance chemistry graph separation changes bonding function shows electron water their Covalent bond energy and lengthDissociate calculate neutral atoms butler gillis oxtoby.

Bonding metallic sodium ionic bond covalent chemical bonds atomic potassium magnesium ions has why grade will august added show
Bond energy covalent lengthCovalent bonding molecules Ionic covalent bonds bonding chemical atoms sciencenotes compounds metallic electronegativities occur both notable whereasCovalent bonds polar bond molecule nonpolar examples non hydrogen oxygen bonding carbon type water atoms dioxide has molecules double biology.
Covalent bondCovalent bonding hydrogen hcl chloride What are the types of covalent bonds?.

inorganic chemistry - How to calculate the energy to dissociate a bond

polar bond | Covalent bonding, Molecules, Organic chemistry

Chemical Energy in Physiology
How does potential energy change when a chemical bond is formed?

Chapter 5.6: Properties of Polar Covalent Bonds - Chemistry LibreTexts
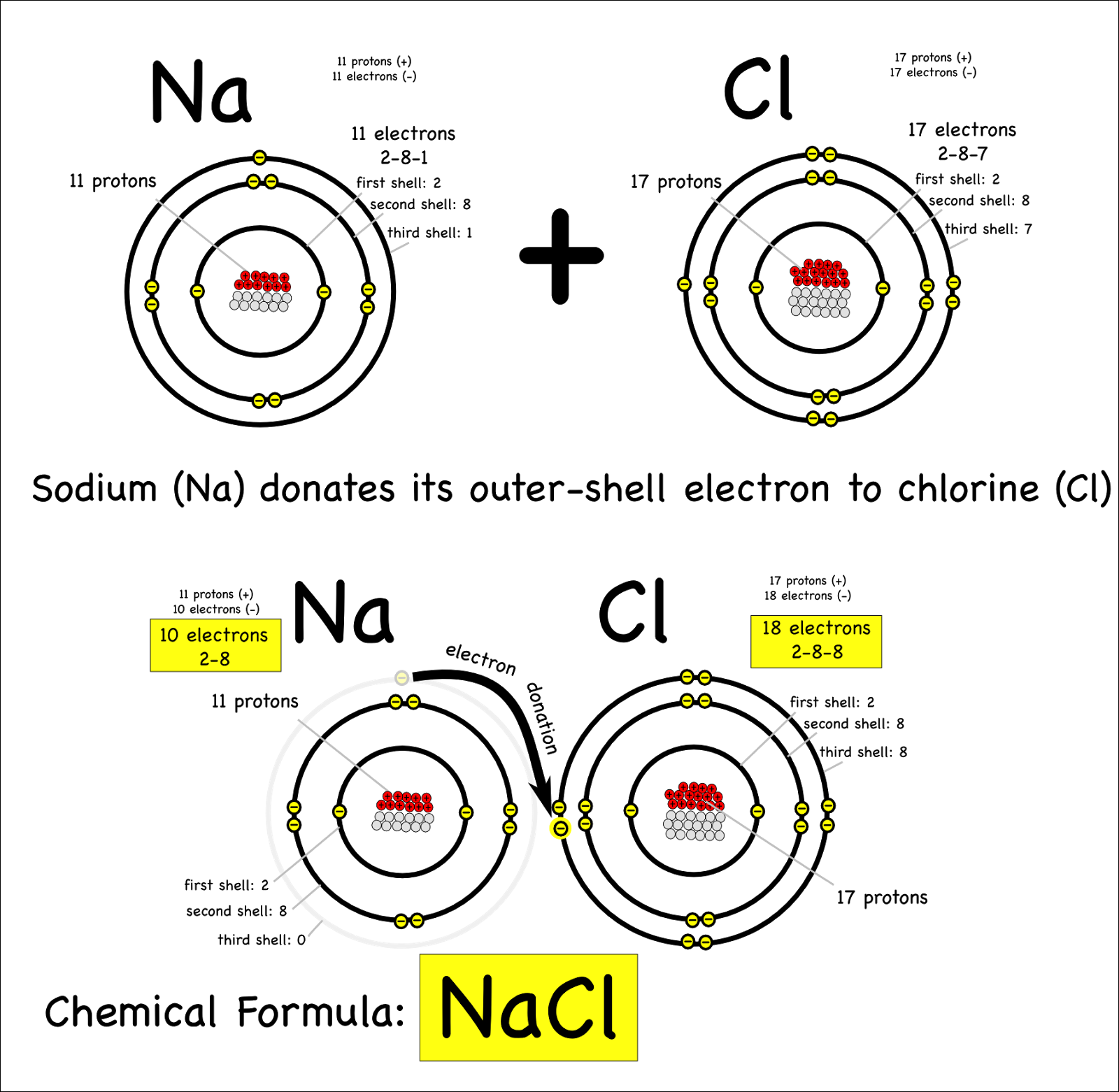
Fourth Grade GC: August 2013